Shortly after the first COVID-19 vaccines received emergency use authorization in the US, survey questions about vaccination status were added to the COVID-19 Citizen Science study. Participants were asked to report monthly if they had received a vaccine, and if so, to indicate brand, along with any side effects that were experienced after the jab.
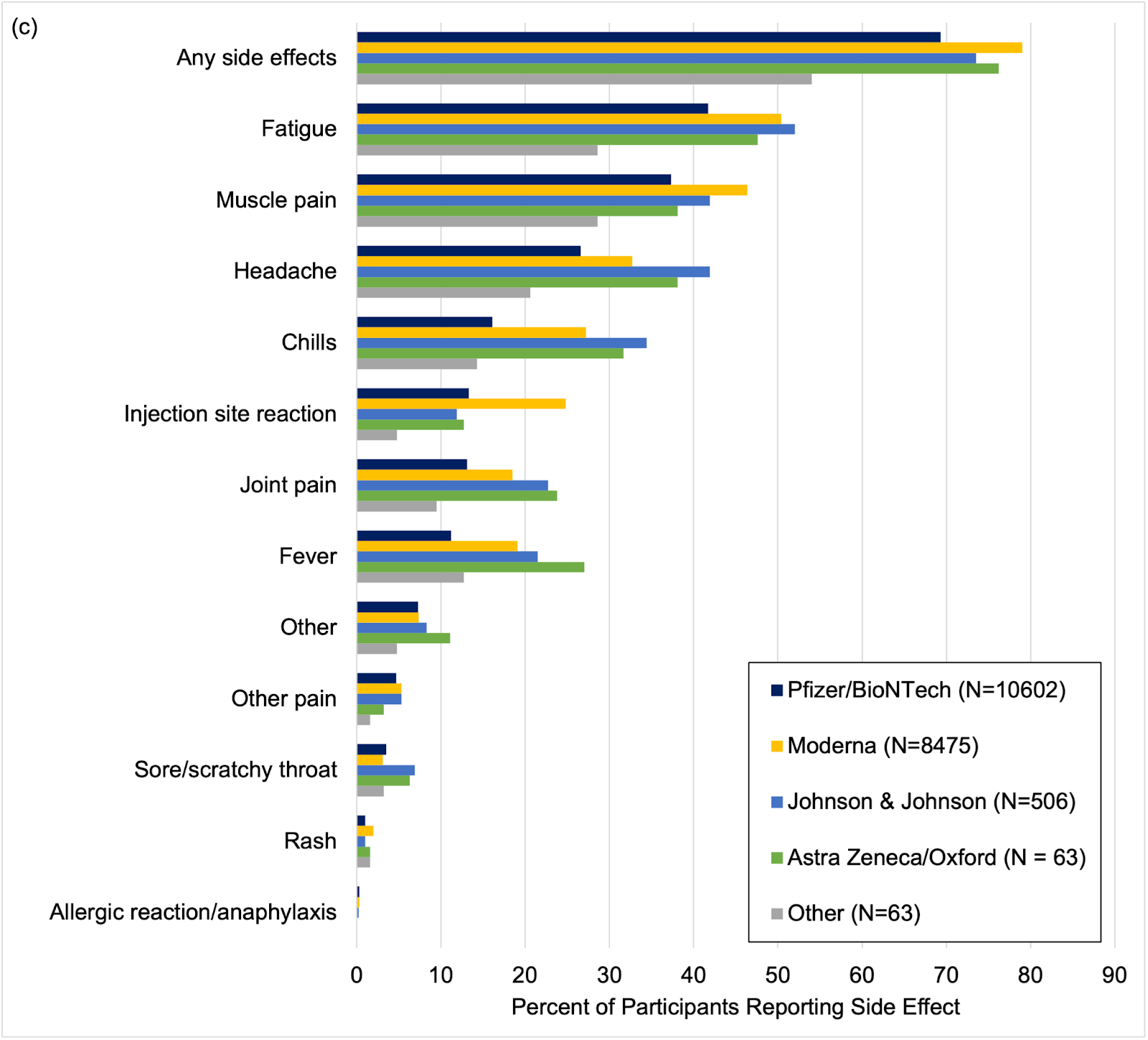
CCS investigator Alexis Beatty, MD, MAS, dug into the study data to see if there were any trends that may be predictors of side effects from COVID-19 vaccinations. As of April 20, 2021, 19,717 study participants had indicated they received at least one dose of a vaccine, of which 73.5% reported at least one vaccine side effect. The participant-reported data showed that side effects from a COVID-19 vaccine were most common with the Moderna vaccine, when compared to other vaccine brands.
Side effects after COVID-19 vaccination by vaccine brand
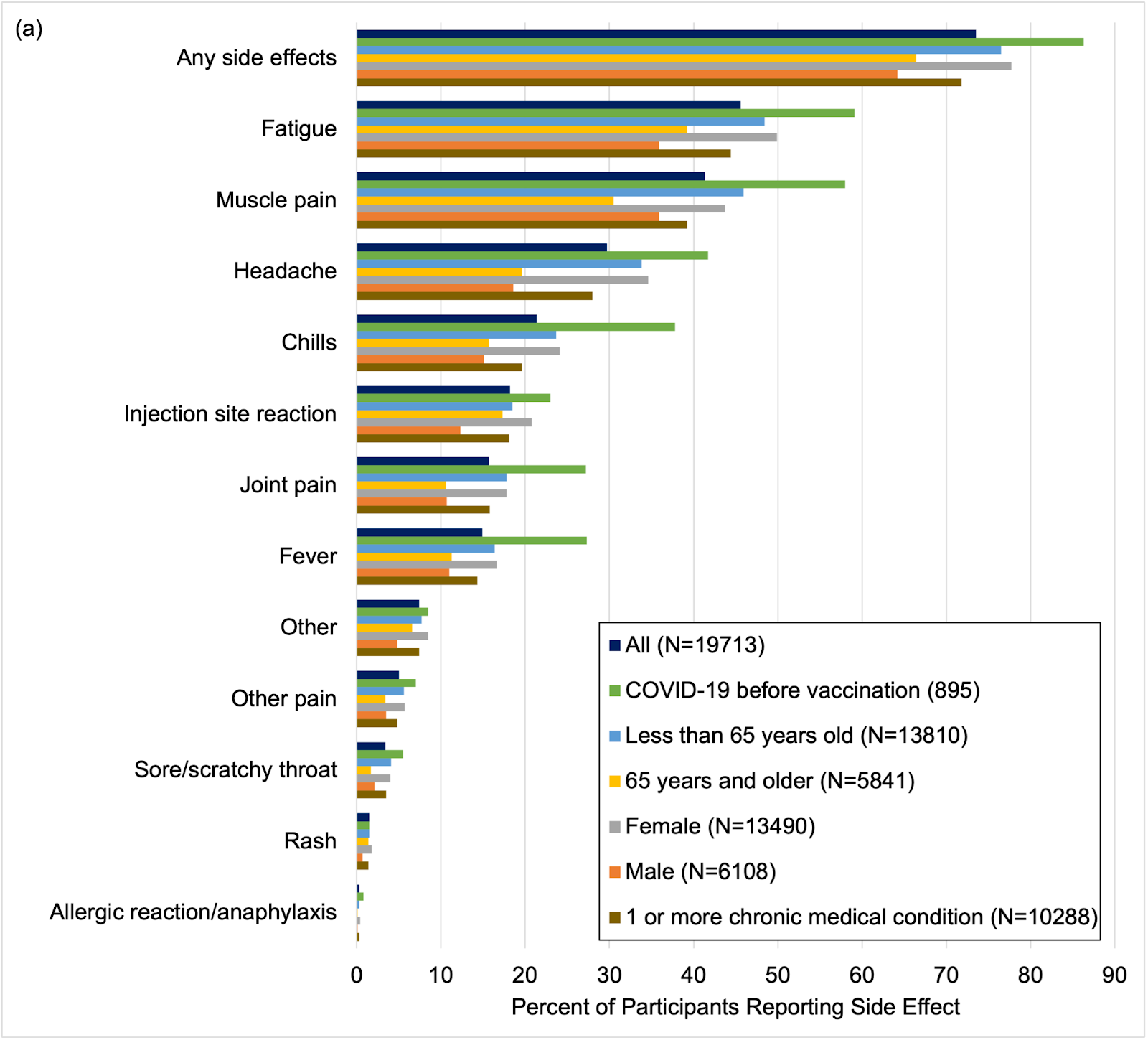
Furthermore, data showed that side effects from a vaccine were more common in younger study participants (less than 65 years old), female sex, and those who reported having COVID-19 in the past. Serious side effects were rare (0.3%).
Side effects after COVID-19 vaccination by participant characteristics
Research into the side effects of COVID-19 vaccines is important because the medical community may be able to identify predictors of more serious side effects. The full article with more findings and figures will be posted here when published!



